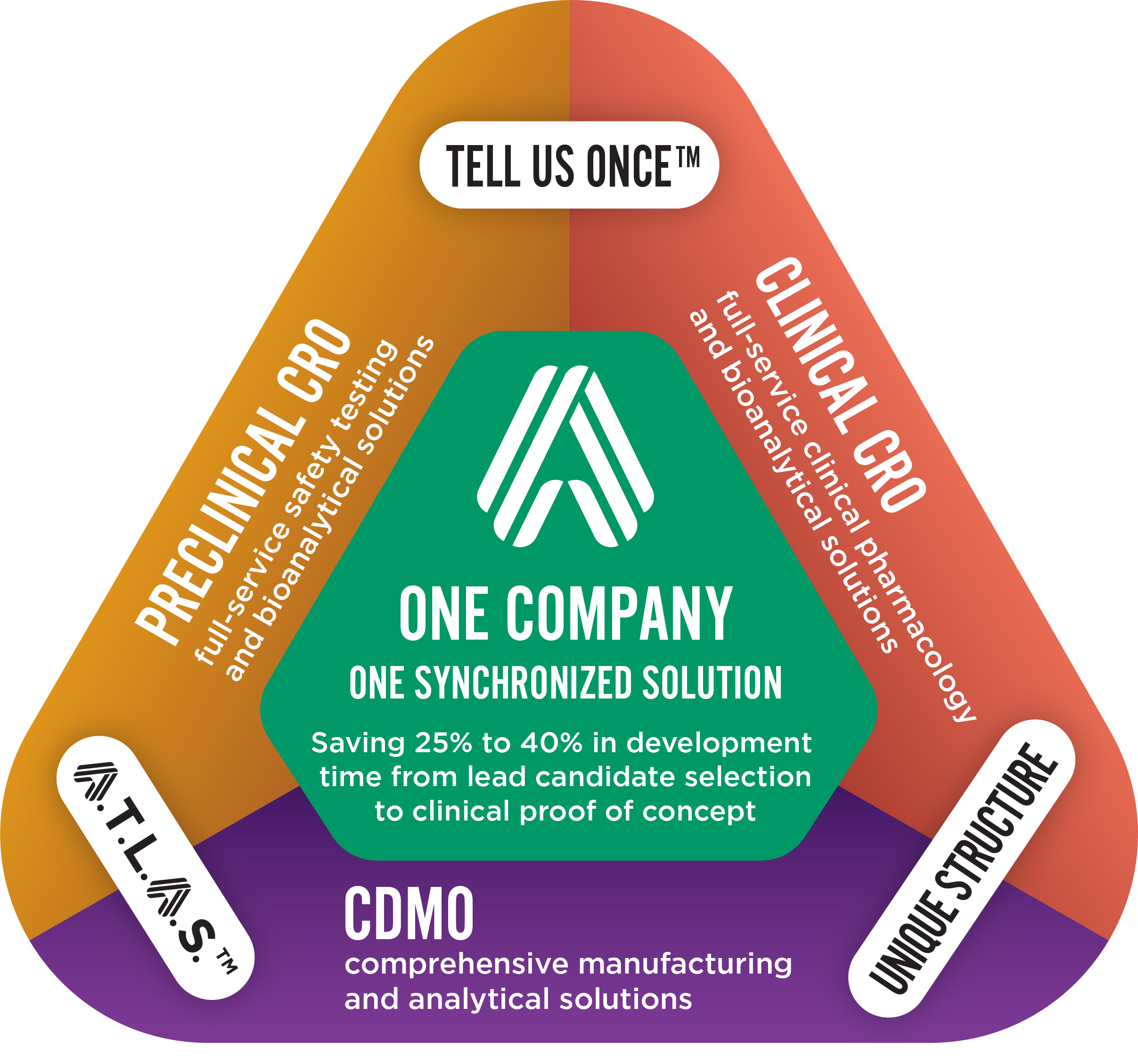
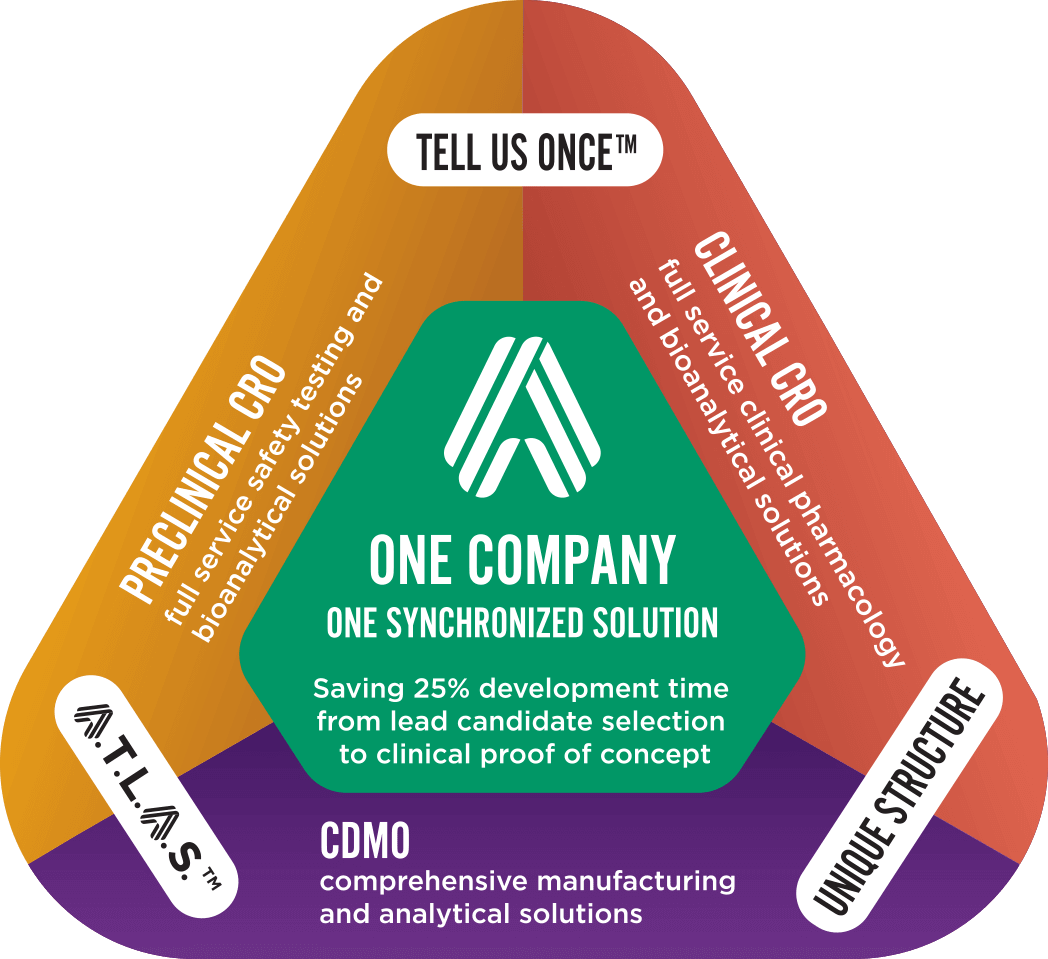
Altasciences’ expertise includes all the clinical pharmacology studies required for regulatory submissions across a wide range of therapeutic areas, covering small molecules, biologics, and 505(b)(2) or hybrid applications.
Our experts will guide you in clinical strategy and ensure proper conduct of your studies — working with you to leverage preclinical data in the design of clinical trials that will take your programs through to proof of concept.
With over 30 years of experience delivering clinical services, we conduct trials in state-of-the-art, purpose-built facilities in the U.S. and Canada, with over 500 beds and a database of more than 400,000 participants (healthy normal and patient populations). Regardless of participant type or length of stay, our recruitment and retention rates are excellent, with 95% on-time panels, year after year.