Expert Ligand Binding Assays for Drug Development
Work with a CRO that offers deep expertise and builds tailored solutions for immunoassay development—and broader bioanalytical methods—plays a vital role in any comprehensive bioanalytical strategy. We leverage a wide range of platforms to deliver high-quality immunoassay services and streamline our operations to ensure our assays scale efficiently with your evolving needs.
We optimize assay sensitivity, apply advanced bioanalytical methods such as ligand binding assays to tackle complex bioanalytical challenges head-on, and uphold the highest scientific integrity to support your drug development program.
KEY LBA APPLICATIONS
Altasciences applies decades of immunoassay expertise to deliver high-quality, reliable data across all phases of assay development, validation, and testing—helping get innovative therapies to market faster. Our scientists handle complex methods and diverse therapeutic areas, including metabolic, neurologic, oncology, genetic, and autoimmune diseases. With experience in a wide range of sample types—from plasma and serum to cerebrospinal fluid and tissues from various animal models—we’re prepared to develop custom immunoassay solutions tailored to your unique project needs.
Our Offering
Pharmacokinetic (PK) and Pharmacodynamic (PD) Studies
- Measure drug concentration over time
- Assess biological responses to treatment
Immunogenicity Testing
- Detect anti-drug antibodies (ADAs)
- Regulatory requirements to determine if immunogenicity against the drug impacts safety and efficacy
Biomarker Discovery and Validation
- Quantify disease-related biomarkers
- Evaluate treatment response
Biopharmaceutical Development
- Support clinical and nonclinical development of monoclonal antibodies, gene therapy products and other biologics
- Evaluate product efficacy and safety
Clinical and Translational Research
- Investigate receptor-ligand interactions
Whether you’re evaluating early-stage candidates or advancing toward regulatory approval, we offer tailored solutions to meet your program’s needs.
COMPREHENSIVE LIGAND BINDING ASSAY TYPES
Our laboratories offer a comprehensive suite of validated assays, supporting diverse research needs from early-stage development to Phase III clinical trials. We optimize each assay type to achieve maximum sensitivity and reproducibility.
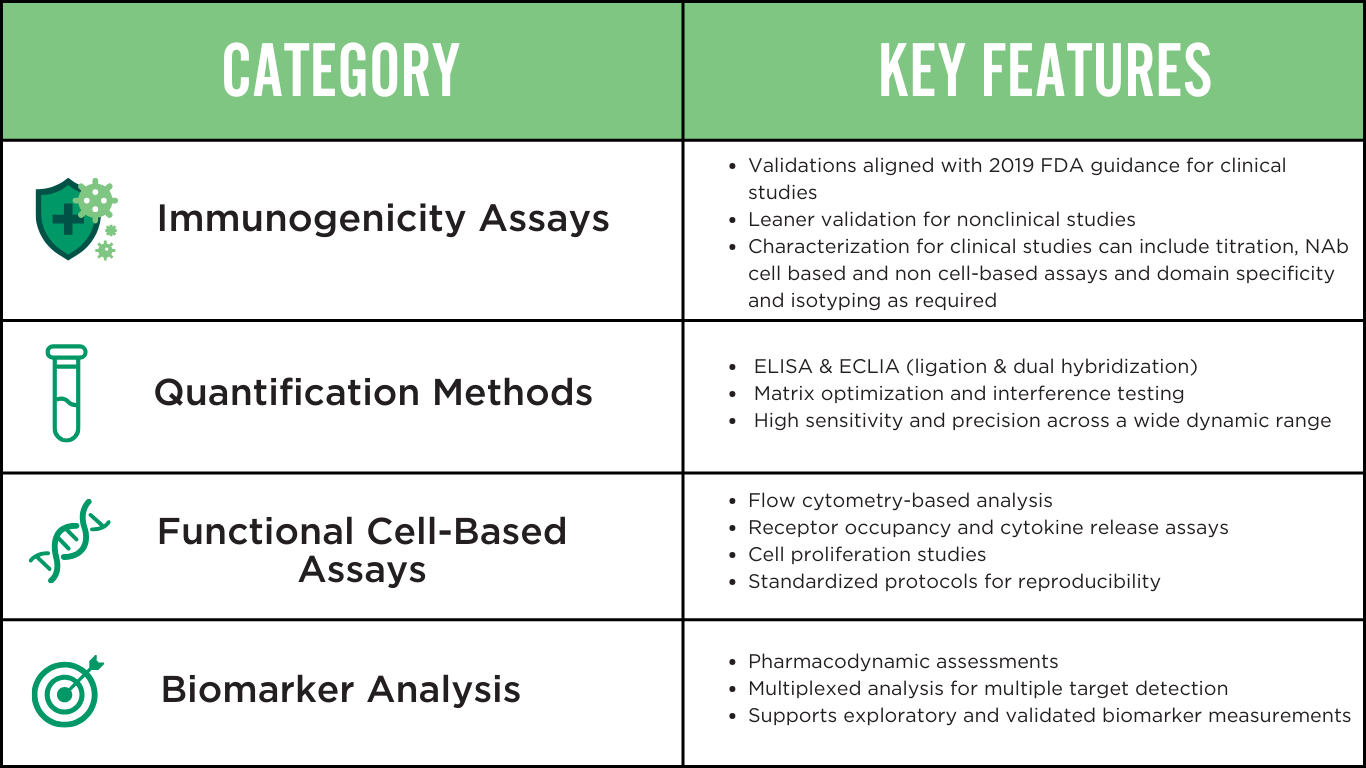
We develop all assays under GLP and GCP guidelines, rigorously validating each one to ensure data integrity while meeting FDA, EMA, and ICH requirements.
CASE STUDY
TAILORED METHODS FOR YOUR DRUG DEVELOPMENT NEEDS
In today’s complex pharmaceutical landscape, successful drug development demands a partner who delivers comprehensive bioanalytical solutions. We support your development journey from preclinical through clinical trials by combining advanced technology with deep scientific expertise in our state-of-the-art facilities.
We tailor our core capabilities to meet your project’s specific needs. Whether you're developing biologics or pioneering new therapeutic modalities, we customize our bioanalytical services to align with your development timeline and regulatory requirements.
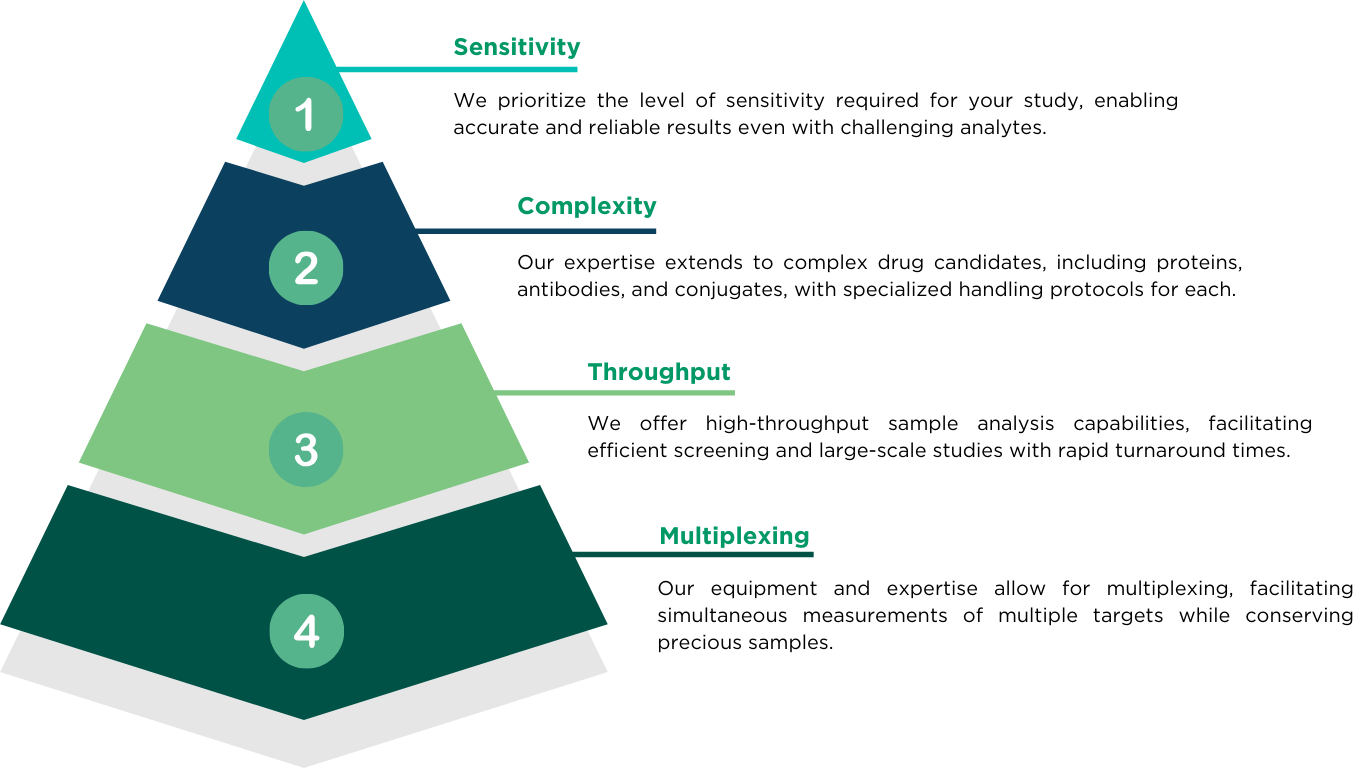
WHY CHOOSE ALTASCIENCES FOR TAILORED LIGAND BINDING ASSAY (LBA) SERVICES
We deliver precise, reproducible ligand binding assay (LBA) solutions across all phases of development—from early research to clinical trials—with the flexibility to support a single study or full program. Backed by decades of scientific expertise, advanced technology, and a commitment to quality and compliance, our tailored bioanalytical services provide reliable data to accelerate decision-making and safely keep your development on track.
To design customized ligand binding assay strategies that support your goals, our experienced team works closely with you.
Here’s what sets us apart:
- Expertise in Ligand Binding Assays (LBA): Delivering accurate, reproducible results supporting data integrity.
- Customized Solutions: Tailored to meet the specific needs of biologics and novel therapeutic modalities.
- Commitment to Quality and Compliance: We ensure data integrity meets stringent regulatory standards.
- Streamlined Development Timelines: We optimize our processes to help you make informed decisions quickly.
- Collaborative Approach: Our experienced team works closely with you to design the most effective strategies for your unique project.
- Integrated Preclinical and Clinical Solutions: Seamlessly supporting both preclinical and clinical stages, with the ability to perform standalone services.
REQUEST AN LBA CONSULT
Partner with experts who bring extensive experience and a commitment to quality, navigating your studies with ease. Our tailored solutions, supported by our flexible bioanalytical services, lay a solid foundation for your drug development program.
OUR COMPLETE RANGE OF BIOANALYTICAL SERVICES
Explore Altasciences' comprehensive bioanalytical services, designed to deliver accurate and reliable data at every stage of drug development. From method development to sample analysis to immunogenicity testing, gene therapy support, biomarker analysis, and more, our expertise facilitates seamless integration with your preclinical and clinical programs, supporting confident decision-making and regulatory success.
Related Resources
View this fact sheet that summarizes our bioanalysis services, which includes both small and large molecules, from the discovery stage, to preclinical, all the way to Phase IV.
This issue of The Altascientist breaks down the complex intricacies of immunomodulation in clinical trials, including the standard classes of immunomodulators, advanced bioanalytical methods, and case studies with practical insights.
In this eBook, we discuss our seamless service offering for small molecule drug development from the discovery stage to Phase IV and beyond.
Check out this eBook which discusses the process of large molecule drug development, and how our full-scale service offering can help streamline the process.
In this eBook we share the importance of monitoring complement factors and cytokines—a case study and nonclinical study outline included.
ANSWERING YOUR KEY QUESTIONS ABOUT OUR LIGAND BINDING OFFERING
Selecting the right partner for ligand binding assays is crucial to your program’s success. Here are some answers to our most-asked questions:
Do you have experience with diverse molecule types?
Yes. Our team has nearly a decade of experience working with:
- Small and large molecules
- Oligonucleotides and peptides
- Proteins
- Monoclonal and bispecific antibodies
- Antibody-drug conjugates (ADC)
Can you develop and validate custom assays for my specific needs?
Absolutely. We have successfully developed and validated hundreds of ligand binding assays, including:
- Hybridized LBA and LC-MS assays
- Tailored solutions for unique therapeutic modalities
- Reliable and reproducible data generation
How do you ensure data integrity and compliance?
Our approach:
- Rigorous GLP and GCP guidelines
- Compliance with FDA, EMA, and ICH regulations
- Comprehensive validation
Do you support both preclinical and clinical stages?
Yes. We provide:
- Early-stage feasibility testing
- Full preclinical and clinical bioanalysis
- Standalone services for seamless integration into your workflow
With our proven track record, technology, and scientific expertise, we are ready to support your ligand binding assay needs—no matter how complex.