
Clinical Research Services for Early Phase Drug Development
Accelerate early‑phase development with focused clinical research—Phase I, Phase IIa, and clinical pharmacology/NDA‑enabling trials. We support small and large molecules across routes of administration and recruit both healthy volunteers and special and patient populations.
Consult our Clinical Services Fact Sheet for details on our early-phase clinical research capabilities.
What Sets Altasciences Apart in Early-Phase Clinical Development?
Progress in early-phase trials hinges on precision, speed, and flexibility. With our focus on early clinical development, we are uniquely positioned to help you progress through critical milestones quickly and reliably. If you are looking for full support and planning of your program to maximize smooth progression, or you have a single study that requires immediate design and execution, Altasciences is the ideal CRO partner.
BEDS
CLINICAL TRIALS
COMPLETED
ANNUALLY
PARTICIPANTS IN OUR
COMBINED DATABASE
Integrated Solutions for Clinical Research Services
We offer a turnkey clinical development solution designed to meet all your needs, whether you require full-service support or something in between. As early-phase clinical research experts, you can expect comprehensive solutions for first-in-human (FIH) trials, clinical pharmacology/ NDA-enabling studies, and proof-of-concept (PoC) studies. Our team handles every aspect of your clinical development, ensuring seamless conduct from start to finish. With our deep expertise, timeline flexibility, and excellent enrollment results, we tailor our services to suit your specific project requirements.
Trust us to provide the guidance, support, and expertise needed to safely accelerate your drug development journey, efficiently.

FIH Trials
We specialize in providing comprehensive FIH clinical research services, guiding you from preclinical development through early-phase trials. Our clinical research experts have extensive experience in designing, conducting, and reporting on FIH studies, ensuring safety, regulatory compliance, and rapid progression to the next phase. With state-of-the-art facilities and expert clinical pharmacologists, we offer tailored solutions that meet the unique needs of each investigational product.

PoC Trials
With a team of highly skilled regulatory experts, bioanalytical scientists, statisticians, medical professionals, and clinical operations specialists, we excel in designing and conducting PoC studies. Our integrated and comprehensive approach allows you to safely reach critical milestones faster and with confidence.

Clinical Pharmacology Trials
We offer expert clinical pharmacology trial services designed to optimize the development of new therapies. Our team specializes in:
Specialized Clinical Capabilities
Within the context of our early phase clinical research services, we have specialized expertise in:
CNS-active drug development requires specialized assessments focused on (among others) cognitive function, dependence evaluation, and abuse potential. Our CNS Center of Excellence has the expertise to advance your clinical program with the most rigorous attention to quality and safety. We offer regulatory guidance, specially adapted clinical spaces for psychedelic trials, and highly trained personnel who ensure the safety and comfort of all participants. We have fully integrated services that will take your program to the next level, including preclinical and bioanalytical expertise.
- Human Abuse Potential
- Physical Dependence
- Eight-Factor Analysis
- Cognition
- CSF collection
- Pain Models
- Driving Simulation
- Psychedelics
 Cardiac Safety Assessments
Cardiac Safety Assessments
We have been designing and conducting QT studies since the FDA E14 guidance was finalized. Our clinical trial services team has experience in both early cardiac safety assessment and thorough QT prolongation studies.
Reach regulatory milestones faster with ethnobridging clinical trials. Altasciences can help reduce your drug development timelines by researching Asian populations where studies have already begun in North America or Europe, according to protocols accepted by Asian countries.
 Imaging and Scans
Imaging and Scans
Altasciences has the experience and affiliated facilities to incorporate imaging into most clinical pharmacology studies.
 PBMC Collection and Separation
PBMC Collection and Separation
Evaluate immune responses and gain a deeper understanding of immune system function pre-dose, post-dose, and at other integral stages of testing with Altasciences’ expertise in routine isolation of peripheral blood mononuclear cells (PBMCs). Through effective harvesting, processing, and analysis of PBMCs, researchers can characterize the human immune response to disease and various immune-targeted therapeutic agents.
 Topical/Transdermal Assessments
Topical/Transdermal Assessments
Benefit from our decades of experience with NDA, 505(b)(2) and marketed TDS products, including narcotics, antipsychotics/CNS therapeutics, hormonal replacement therapies, nicotine, analgesics, immunologicals, and more. We are fully versed on FIH, NDA and ANDA study requirements for North America and Europe, and can advance your topical research efficiently and effectively.
 Opthalmological Evaluations
Opthalmological Evaluations
Ophthalmology safety or efficacy assessments can be performed on site or by leveraging our partnerships with local specialty clinics in healthy participants or patients. Our Montreal facility has an ophthalmologist Principal Investigator who performs assessments both at his office and our clinic, located in the same building.
The lungs are an excellent target for drug delivery, both as the end organ for the treatment of respiratory diseases and as the route of administration for systemic therapies. The lungs provide direct access for treatment of respiratory diseases, while providing an enormous surface area and a relatively low enzymatic, controlled environment for systemic absorption of medications.
We offer expertise in multiple modes of inhaled drug delivery, including nebulizers, metered-dose and dry-powder inhalers, nasal sprays, and other devices. We have conducted trials on these drugs from FIH to ANDA. Our clinical research services utilize purpose-built inhalation/specialty negative pressure rooms for the dosing process to ensure a safe and contained environment for participants and staff.
Efficient Study Start-Up and Clinical Research Services Timelines
We pride ourselves on our ability to mobilize quickly. For instance, we recently reduced the start-up time from the industry standard of 12 weeks to less than four weeks. Our approach includes effective communication, strategic planning, and a dual project management structure, ensuring seamless alignment between our internal teams and the sponsor. This facilitates prompt decision-making and rapid issue resolution.
By streamlining processes—such as contract execution, regulatory document preparation, and subject recruitment—Altasciences demonstrates a commitment to efficiency and timely results. Our strong project management, combined with a dedicated internal team and collaborative relationships with sponsors, leads to significant reductions in start-up timelines.
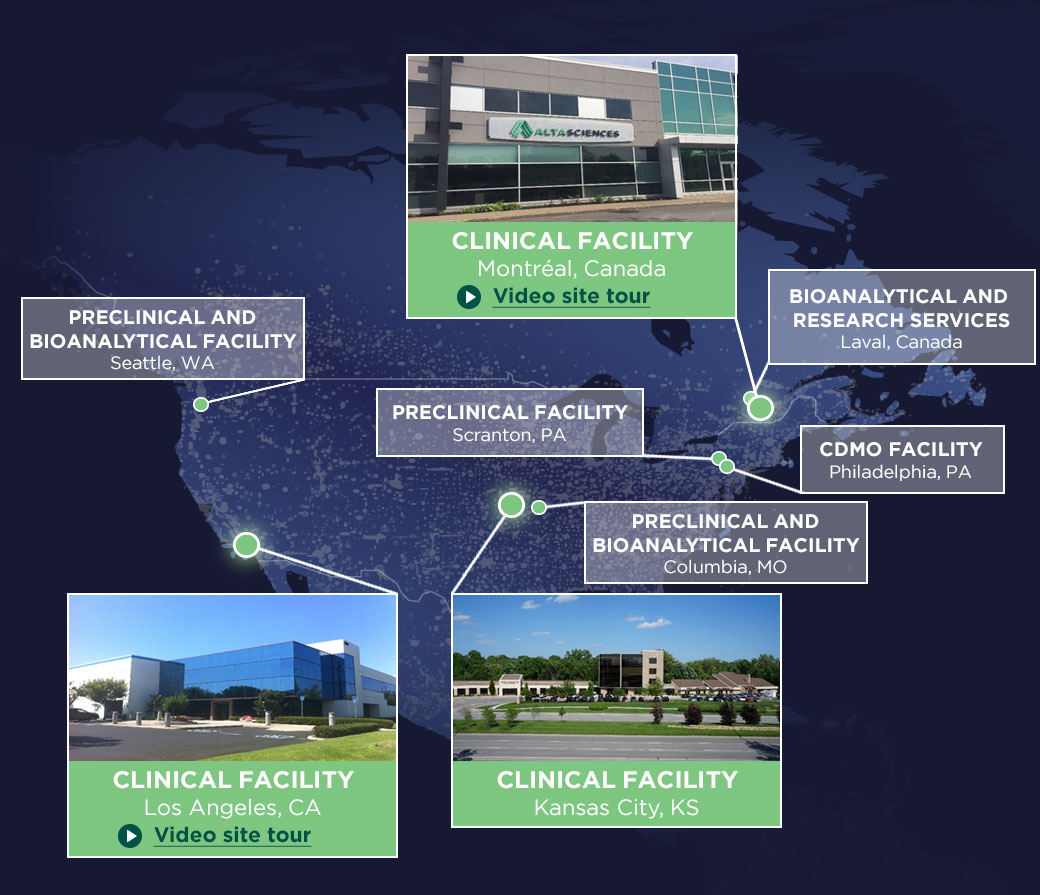
With three clinical pharmacology units and co-located bioanalytical laboratories in the U.S. and Canada, we provide multiple regulatory pathways—Investigational New Drug (IND) and Clinical Trial Application (CTA) in Canada and the EU. Our focus on efficiency ensures rapid start-up and streamlined timelines for your projects.
Optimized Recruitment Strategy and Rapid Enrollment
With a pool of over 400,000 healthy normal participants, special populations and patients, we rapidly recruit and enroll full panels. Our recruitment strategies and database ensure we meet enrollment targets swiftly, so your study progresses without delays.
Comprehensive Clinical Research Services
We offer a wide range of services for conducting early-phase clinical trials and will manage every aspect of your trial from start to finish. Our comprehensive services include:

Program/Project Management
Our program/project management solution is tailored to navigate the complexities of clinical research services. We excel in managing diverse study designs across a wide range of therapeutic areas, delivering customized solutions to meet your specific early-phase drug development needs.
With decades of expertise in the entire early-phase drug development spectrum—including preclinical studies, clinical trials, manufacturing and analytical services, bioanalysis, and complementary CRO services—our team also ensures comprehensive management for large-scale, end-to-end programs.

Protocol Review and Development
Our approach focuses on efficient timelines and precise data collection. Whether starting from scratch or refining an existing protocol, we ensure a streamlined process to support informed decision-making and meet critical study milestones with regulatory-compliant, customized protocols.
Early-Phase Clinical Conduct
Our three state-of-the-art clinics across North America are fully equipped to conduct early-phase trials. Our expert teams manage all aspects of your trials with adherence to the highest quality and safety standards.

Clinical Research Associate (CRA) and Clinical Monitoring
We provide timely tracking of pharmacokinetics (PK) and pharmacodynamics (PD) data, safety assessments, and adverse event reporting. This ensures regulatory compliance and maintains data integrity throughout the trial.

Bioanalytical Services
Our co-located, state-of-the-art bioanalytical labs will develop and validate methods specific to your clinical trial. We also have over 685 validated methods for a wide range of compounds. By leveraging these established assays, we deliver precise and timely PK data to meet regulatory standards. This enables faster decision-making and comprehensive label information.

Data Services and Reporting
We manage data collection and analysis, and provide comprehensive reporting to deliver reliable, well-organized, and actionable, early-phase clinical trial results—on time.

Regulatory Consulting
Our experts provide guidance on when and how to conduct your early-phase clinical trial, helping you map out a clear, efficient path through the complex regulatory process.
Consult our Integrated CRO Services fact sheet.
Purpose-Built Phase I Clinical Research Units
Our Phase I clinical trial units in the U.S. and Canada offer you over 580 beds, and:
- Dedicated participant screening facilities
- Long-term stay facilities
- Outpatient and return units
- Suite of 12 on-site driving simulators, with space for 20 more
- Customized facilities for in-patient psychedelic settings
- Secure pharmacies with video monitoring and retinal scanning
- Pharmacists experienced with complex and narcotic compounding
- Access to co-located ophthalmology clinic
- Co-located bioanalytical laboratories
Commitment to Safety
Our clinical facilities are under 24/7 video surveillance, have controlled access throughout, and are near major hospitals. Full-time research physicians oversee all aspects of our early clinical development studies. Trial participants are assessed daily by an Investigator and are under constant supervision. When needed, we have a telemetry system to monitor ECGs and pulse oximetry. There are also strategically placed panic buttons throughout our clinics, and Advanced Cardiac Life Support (ACLS) provider coverage on-site 24/7. In addition, all clinical staff are certified in Basic Cardiac Life Support and trained in scenario-based response.
THERAPEUTIC AREAS
Our deep expertise and capabilities in a broad range of therapeutic areas encompasses preclinical and early clinical studies for both small molecules and biologics. We can manage your entire program, as well as provide comprehensive support research services and bioanalytical expertise.
Related Resources
Consult our Clinical Services Fact Sheet for more information on our clinical trial service capabilities.
Consult our Pharmacy on Demand Fact Sheet to see how we save you time with integrated processes and synergies between manufacturing and clinic.
Consult our Clinical Sample Kits Fact Sheet to learn more about our streamlined sample management process, and comprehensive kits.
Early Phase Clinical Research Services–FAQs
What clinical research services are available?
Altasciences provides fully integrated regulatory, scientific, and strategic guidance, protocol development, clinical pharmacology and monitoring, state-of-the-art bioanalysis, expert project management, and CDISC-compliant data management. We also have highly skilled teams in biostatistics and medical writing/reporting. Whether you are looking for a single study or a full program, our clinical CRO services can simplify the process for you.
Does Altasciences offer CDMO services for clinical trials?
Yes. In fact, Altasciences’ integrated teams’ combined expertise in clinical trials and manufacturing simplify the drug development process, empowering sponsors to bring innovative therapies to market faster and, ultimately, improve patient outcomes. This cohesive approach minimizes delays, reduces costs, and enhances collaboration. Our CDMO team offers drug development, manufacturing, and analytical services, including formulation development, Phase I through large-scale commercial manufacturing, and ICH stability storage and testing, to pharmaceutical and biotech companies worldwide.
Why is Altasciences a trusted CRO partner?
Altasciences is a mid-size, forward-thinking CRO with all the scientific expertise and experience to successfully manage your early-phase drug development program, with a fully integrated preclinical to clinical offering, including expert bioanalysis, manufacturing, and analytical services.
How do you ensure that your clinical research services provider has a quality culture?
Ensuring that a CRO treats the quality of their work with the utmost care is crucial. At Altasciences, quality is our primary driver, for clinical research services, and every other service offering we have, from development to bioanalysis to manufacturing. Ensuring that we are always aligned with the guidelines and that our interactions with regulatory agencies are productive are critically important.
How does Altasciences ensure an integrated approach for their comprehensive services?
Our vision is to provide sponsors with a seamless drug development process, from lead candidate selection through to proof of concept, and beyond. We provide the scientific, strategic, and regulatory expertise to guide you through each development phase. With Altasciences as your only CRO partner, integrated, comprehensive, and scientifically advanced, you only need to Tell Us Once™ about your program goals, and we’ll take care of the rest.
How much industry experience does Altasciences have?
Our company has been thriving for over 30 years, consistently delivering top-quality clinical research services to sponsors worldwide, in addition to comprehensive preclinical services, bioanalysis, manufacturing, and analytical services. Our teams offer vast and broad industry experience from CROs, biopharma and pharma, academia, government, and other industry sectors.






