CRO Services
Altasciences has been providing clinical research support services to the global biopharmaceutical industry for close to three decades. Our expert teams are fully equipped to support, manage, analyze, and report on studies conducted either here at Altasciences or with external collaborators. Whether as part of a development program or a single study, our research support teams deliver all the complementary clinical research support services needed to complete your projects.
Consult our comprehensive clinical research support services fact sheet
Benefit from Working With a Full-Service CRO
As a full-service CRO, we proactively manage your early-phase to Phase Ib/IIa clinical trials from start to finish. Benefit from a single partner across early phase with project-specific services and focused clinical study management, as well as a single point of contact with the expertise to manage all aspects of your clinical trial.
CRO Services
Integrated Services
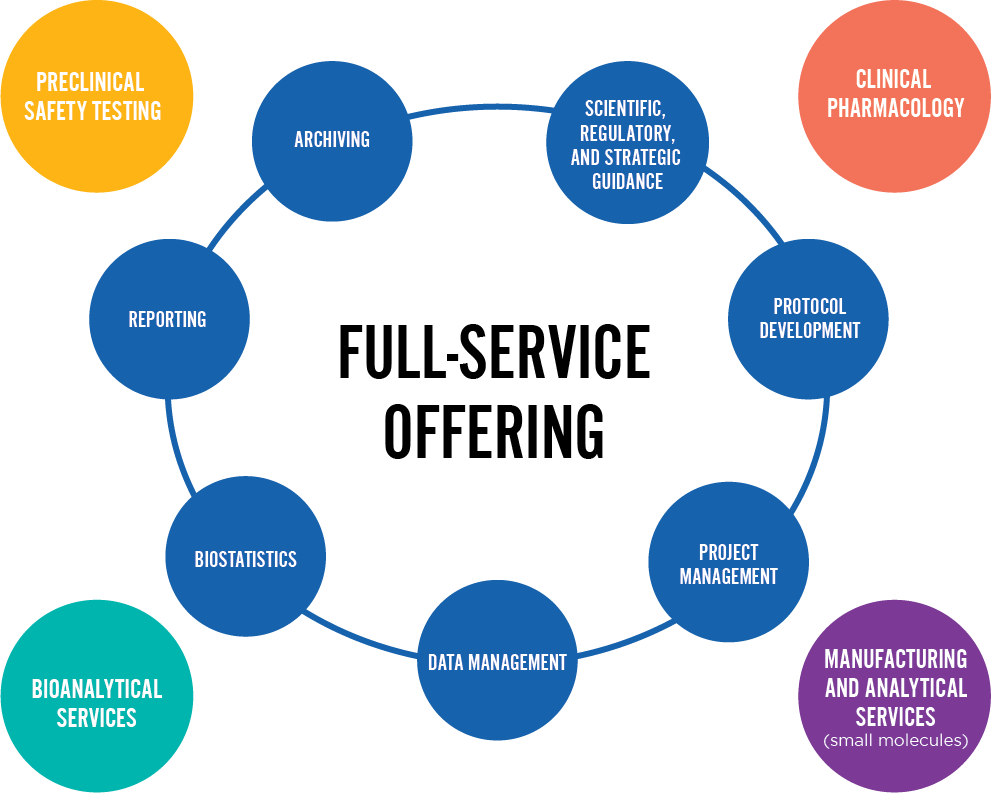
Clinical Research Support Services
The evolving landscape of drug development continues to reshape our world. Simultaneously, clinical research has become notably more challenging with its share of scientific, operational, and regulatory complexities. Our mission is to support you in safely expediting the development of your therapeutics. We are experts at orchestrating a spectrum of services for optimal accountability, efficiency, and integration. With Altasciences, you can expect quality outputs and efficient operations, as well as less duplication of management oversight on your end.
Our approach synchronizes operational proficiency, medical insights, regulatory knowledge, and the resources of our nonclinical sites, wholly owned laboratories, Phase I units, and manufacturing site. This integrated solution is designed to streamline and enhance your journey, providing an integrated early-phase pathway for accelerated drug development.
CRO Services - FAQs
Are Your CRO Services Flexible?
Our comprehensive CRO services are entirely adaptable to your needs. You can partner with us for individual, stand-alone services, coupled with other third-party work, or include them as part of a full drug development package exclusively with Altasciences.
Will You Work With Our Third-Party Vendors to Deliver CRO Services for Studies Conducted Elsewhere?
Yes. We work with third-party vendors and manage those relationships.
How Do the CRO Services Fit in With Altasciences’ Overall Offerings?
We synchronize and integrate CRO services to provide support at every stage of your program, from formulation development through preclinical to clinical proof of concept, and beyond. With Altasciences, you can work with only one supplier throughout the complete lifecycle of your program, saving you time and effort, and getting your drug to market, faster.
What Are Some of the Specific CRO Services You Offer?
Our comprehensive CRO services include regulatory and scientific guidance, protocol development, clinical and anatomic pathology, specialized chemistry, project management, clinical monitoring, medical writing, biostatistics, data management, CDISC and SEND. All the complementary services to support your programs are available at Altasciences.